Angiogenesis
The majority of human eye diseases in industrialized countries is a result of an abnormal retinal or choroidal vasculature, which leads to a severe or complete loss of vision. Diseases such as age-related macular degeneration, diabetic retinopathy, retinopathy of prematurity or neovascular glaucoma are caused by retinal blood vessel abnormalities including macular edema, retinal and vitreal hemorrhages, as well as fibrovascular scars and membranes. Therefore, knowledge and understanding of the molecular pathways regulating the processes of blood vessel formation and maintenance in the retina are extremely important and represent a prerequisite to develop efficient therapeutic strategies to treat these devastating human diseases and prevent visual loss in affected patients.
Retinal vascularization begins in the most superficial retinal layers (ganglion and nerve fibre layers) at the optic nerve head and radiates outwards from this central point. It reaches the retinal periphery just before birth in humans, and during the first week of life in mice. Additional capillary networks in deeper retinal layers then arise by sprouting angiogenesis (the formation of new vessels from a preexisting vascular structure).
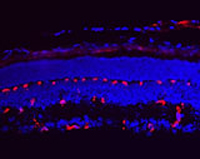
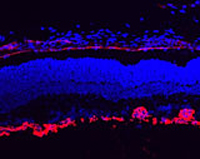
Retinal angiogenesis is a developmental process that leads to the formation of dense vascular networks in the inner retinal layers. These networks of arteries and veins are essential to provide oxygen and nutrients to the different cell types in the inner retina including ganglion cells, bipolar as well as horizontal and amacrine cells. To elucidate the molecular cues of the key mechanisms in retinal angiogenesis, we make use of a mouse model (Norrin knockout mice) with a defect in retinal sprouting angiogenesis. In normal development, the three vascular networks (superficial, deep and intermediate) of the retina are differentiating between postnatal days 3 till 17. Our detailed morphologic and molecular analyses of Norrin knockout mice have revealed an abnormal superficial network and a complete lack of the deep and intermediate plexuses. The presence of aneurysm-like lesions, derived from the scarce and dilated superficial vasculature, suggest a defect in sprouting angiogenesis, as is depicted in the images on the right.
TEAM MEMBERS
Wolfgang Berger (PhD)
Silke Feil
Samuel Koller (PhD)
COLLABORATORS
Ulrich Luhmann (PhD), Institute of Ophthalmology, UCL, UK